See also
( Sorry now 14 )
Facts and Figures
.
Nitrogen Trifluoride Now Required in GHG Protocol
Greenhouse Gas Emissions Inventories – Nos. 8 & 9
.
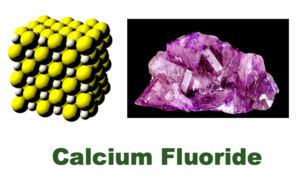
1) Calcium fluoride (CaF2) occurs naturally in some underground waters.
[ Also found on the planet Mars. ]
See ⇒ HERE
[ The Drinking Water Standards were established on this much
less toxic Calcium fluoride which is listed as a moderately
toxic compound compared to hexafluorosilicic acid,
which is categorized as extremely toxic, see below. ]
Calcium fluoride is NOT used for
artificial water fluoridation.
It is not an industrial waste looking for a market ! – Calcium fluoride occurs naturally in some waters and is a health hazard in many countries, as can been seen in the documents on ‘Chronic Endemic Fluorosis Of Merino Sheep In Queensland‘ – (Sheep drinking artesian water). Calcium fluoride is an insoluble ionic compound composed of Ca2+ and F. ions. It occurs naturally as the mineral “Fluorite” – also called fluorspar.
See also ⇒ India
See ⇒ * * * ⇐ below
 .
.
2) Sodium fluoride (NaF) is an inorganic compound.
It is used in F. tablets and the fluoridation of drinking water, also in metallurgy, and as a flux,
and is also used in pesticides and rat poison.
Sodium fluoroacetate – 1080
3) Sodium fluoroacetate ↔ 1080, ↔ Wikipedia, ↔ USA,
See also: Fluorides & Plants – ( Botanical )
4) Fluosilicic acid
(H2SiF6 or F6H2Si) Hexafluorosilicic acid and hexafluorosilicates are the most commonly used agents in drinking water fluoridation and it has been claimed that incomplete dissociation of these agents in drinking water may result in human exposure to these chemicals. The toxicology of these compounds is incompletely investigated. [Corrosion of water mains and home plumbing is a financial and toxic consideration.]
also:
Sodium fluorosilicate
(Na2[SiF6])
5) Hydrofluorosilicic acid
(H2SiF6) also written as (H3O)2[SiF6].
(Disodium hexafluorosilicate, – Sodium silicon fluoride, – Sodium silicofluoride).
Unlike the fluoride compounds found in toothpaste or supplements, these fluoridation chemicals are NOT pharmaceutical grade quality and ARE contaminated with heavy metals e.g. – fluosiloxanes, aluminium, iron, arsenic, beryllium, cadmium, nickel, lead, mercury, chromium, sulphides and radionuclides, –
THEY ARE IN MOST FLUORIDATED DRINKING WATER.
.
– Typical analysis of contaminates:
.
.
6) Hexafluorosilicic acid. ( H3O)2[SiF6 ] )
(See also: Hydrofluosilicic Acid, Hydrtosilicofluodie Acid, Silicofluoric Acid, and Fluosilicic Acid. The toxicology of these compounds is incompletely investigated.)
It is a colourless liquid mostly encountered as diluted aqueous solution. It is manufactured as a co-product in the production of phosphate fertilizers. The major use of sodium hexafluorosilicate acid is as a fluoridation agent for drinking water.
It is also produced naturally, on a large
scale in volcanoes.
See also ⇒ HERE ⇐ Very comprehensive
.
7) Sulfur hexafluoride (SF6) is the most potent
greenhouse gas that it has evaluated, with a ‘g’ of 22,800 times that of CO2 – NASA – (More) when compared over a 100-year period. More than 10,000 tons are produced per year.
8) Both Hydrofluoric acid, (HF) & Nitrogen trifluoride, (NF3) have characteristics that must be managed for safe use and release. HF is a highly toxic and corrosive gas that can have significant health consequences if an individual is exposed to it.
HF is also a highly reactive gas that can react with certain metals to release toxic fumes or explosive hydrogen.
Many organo-fluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. [CO2 is the recent safe replacement used as a refrigerant.]
9) Nitrogen trifluoride (NF3) with a global warming potential of 17,000 times the global warming potential of CO2
It is now present in the atmosphere at four times the expected level and rapidly rising. NF3 is toxic, and is only reactive at high temperatures and when exposed to certain physical conditions. Nitrogen trifluoride appears as a colorless gas with a moldy odor. Very toxic by inhalation. Slightly soluble in water. Corrosive to tissue. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Used to make other chemicals and as a component of rocket fuels.
It is a gas used in flat-screen TV manufacture.
‘
10) Uranium hexafluoride (UF6), is a compound used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.
More  HERE.
HERE.
.
11) Potassium titanium fluoride
More  HERE – Harmful if swallowed.
HERE – Harmful if swallowed.
May cause an allergic skin reaction.
Causes serious eye damage.
.
P.S. – Three More Fluorides:
12) Sulfuryl fluoride (SO2F2), is just the latest of several unexpectedly potent greenhouse gases to pop out of the woodwork. The common factor appears to be fluorine.
It is neurotoxic and a potent greenhouse gas, but is widely used as a fumigant insecticide to control termites.
Last year, for instance, it emerged that nitrogen trifluoride, a gas used in flat-screen TV manufacture, has 17,000 times the global warming potential of CO2
.
13) In 2000, researchers analysing samples of Antarctic snow and air discovered contamination with trifluoromethlyl sulphur pentafluoride, (SF 5CF 3), and Here, which turned out to be 18,000 times more potent green house gas than CO2 per molecule.
It is a rare industrial gas. –
..
14) Trichlorofluoromethane, and Here, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colourless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth’s protective stratospheric ozone layer.
.
Naturally Occurring Radioactive Materials (NORM)
Technical Report Series No. 161
The naturally occurring radioactive elements uranium and thorium, like most chemical elements, are formed in stars and comprise a small amount of the material that formed the earth.
The radioactive isotopes uranium-238 (U-238) More and thorium-232 (Th-232) have decay times (half-lives) which are comparable with, or larger than, the age of our Earth, so they have always been present in the Earth’s crust and within the tissues of all living species…
Full original text  HERE
HERE
![]()
The industrial production of fluorine gas for
uranium enrichment, its largest application,
began during and for the Manhattan Project.
![]() .
.
See also  Radiocarbon dating
Radiocarbon dating
Note: Since atom bomb testing this
may need to be updated as
the tests have increased
radioactive carbon in
our atmosphere.
*
“The lethal dose of NaF
(an artificial fluoride)
is 50 times smaller than
CaF2 the naturally-occurring
Calcium fluoride.”
~ Dr. Hardy Limeback.
Biochemist & Professor of Dentistry,
University of Toronto,
Former Consultant To The
Canadian Dental Association.
 .
.
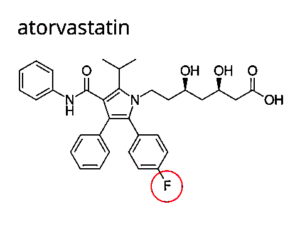
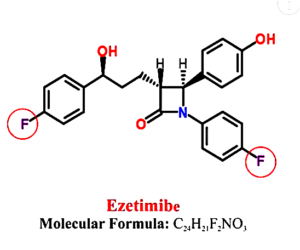
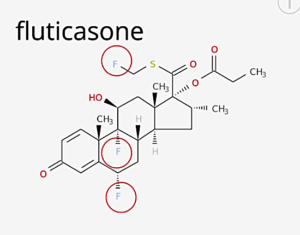
The Truth About Fluoride And Drugs
That Contain Fluoride
The sun is the major driver of the Earth’s climate system
⇒ NASA says CO2 is a coolant
NOT a warming gas ⇐
~ MORE ~
MAJOR DANGEROUS MEDICATIONS (F.)
⇑ A New Very Short Informative Video ⇑
Notice all the “Fs” for fluoride ⇓
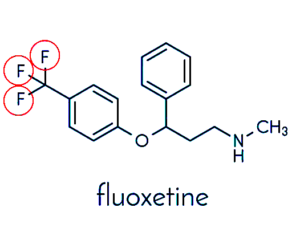
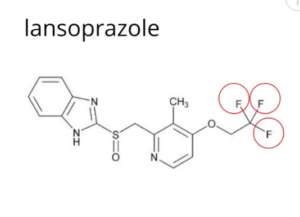
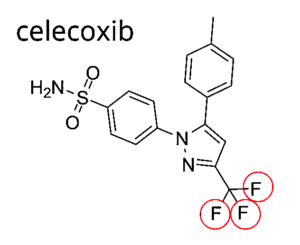
Scientists estimate that, worldwide,
termites may release over 150 million tons
of methane gas into the atmosphere annually.
Also
more CO2 each year
than all living things combined !
‘.
![]()

![]()