24 December | FLUORIDATION & LEAD, RESEARCH PAPERS - B | admin
FLUORIDATION AND LEAD CONTAMINATION.
For millennia lead has held a deep attraction for Painters,
Builders, Chemists and Winemakers – but it’s done
untold harm – especially to children.
This is important interesting, with amazing
history of human tragedy.
A number of factors are involved in the extent to which
lead enters the drinking water, including:
1) the chemistry of the water (acidity & alkalinity) and the types and amounts of minerals in the water,
2) the amount of lead it comes into contact with,
3) the temperature of the water,
4) the amount of wear in the pipes,
5) how long the water stays in pipes, and
6) the presence of protective scales or coatings inside the plumbing materials.
7) The levels of fluorides in the water and the rate of flow.
Regarding the matter of lead being leached from old pipes. Anyone who argues that fluoridation had nothing to do with it will have to explain those well-preserved lead pipes from more than 2000 years ago in unfluoridated Roman water…
Lead in Aviation Fuel & Bullets
FLUORIDE, TOOTH DECAY AND LEAD
Original → HERE ←
The fluosilicates are the by-products of the phosphate fertilizer industry.
In the manufacture of this kind of fertilizer, phosphorus is obtained from
phosphate rock, which has to be broken down with sulfuric acid.
(1) Fluorine occurs naturally in combination with the phosphates.
(2) In these two facts lie the keys to the presence of lead in the fluosilicates.
Step One: Sulfuric acid is prepared by either of two ways, the lead chamber process
(3) or the contact method.
(4) In its purest form (made by the contact method) it is used in pharmaceuticals; in its lowest grade (produced by the lead chamber process) it is used by the fertilizer industry.
(5) It is also frequently recovered for re-use, but this form is too impure for any purpose except the manufacture of fertilizer, for which it is quite suitable.
(6) In the lead chamber process purification is carried out only to the extent of removing substances that could clog the machinery.
(7) Of the common metals, only lead is resistant to cold sulfuric acid in concentrations up to 100%. But in hot acid the resistance is up to about 70%.
(8) The lead chamber type uses heat (about 600 C) and isn’t cooled during the process. That’s why a certain amount of lead is leached during this procedure.
If a pure product is needed, the contact method is used, but it’s more expensive, more complicated. In the making of fertilizer, however, a pure grade is not necessary. After all, neither fertilizer nor its by-products were intended for human consumption.
Step Two: Fluorine, which is a highly reactive element capable of joining with any other element except oxygen [and Neon], is able to leach lead from the contaminated sulfuric acid. In the past hydrofluosilicic acid was simply neutralized and discarded. The picking up of lead wouldn’t have been a problem. But eventually it was decided that the acid, being already in solution, would be better, simpler to use, and less expensive than sodium fluoride.
(9) The lead contamination, apparently, was forgotten (if, indeed, it had ever been noticed.) Sodium fluoride is also lead-tainted (and with arsenic, as well.) Aluminum ore (bauxite) is usually contaminated with lead and arsenic (and a number of other elements.)
In order to obtain a pure product, these have to be removed.
(10) They become part of the major by-product of aluminum refining, sodium fluoride. Another way in which fluoridation contributes to lead in the water is through its action on whatever lead pipes may still be in existence in older homes. Any lead pipes would be old lead. These are ordinarily covered by a protective coating made by the lead itself which is impervious to diluted acids (as all of them would be in water.) Water acts slowly on lead, forming lead hydroxide, but the action is slight if the water contains carbon dioxide or carbonates or sulfates which interact with lead to form these protective coatings.
(11) It’s interesting that the lead pipes in Roman aqueducts, 2000 years old, are still in such good shape the numbers and letters engraved on them are clearly legible. [See image below.]
(12) In fluoridated water, though, it’s a different matter. Fluorine can and does destroy the protective coatings; it can and does leach lead.
A pediatrics textbook published in 1964
(13) noted that the incidence of lead poisoning had been rising in certain metropolitan areas in Eastern United States. The blame was laid on old lead paint flaking from walls and woodwork. But most of the lead chips were old before 1964; some children chewed them long before then. But a new source of lead had arisen–unnoticed: The fluoridation of water, with lead-contaminated fluoride, a substance also capable of leaching lead from the pipes. Although there were scattered places fluoridating throughout the nation, larger numbers of eastern metropolitan communities were doing so. Today one in nine children under the age of six is said to have unacceptably high blood lead levels
(14) even though lead paint was banned in 1978 (and hadn’t been used extensively since the 1950’s!) Lead in gasoline has been phased out, [except in aviation fuel] and lead solder hasn’t been permitted on copper tubing since 1986 (eight years ago.) The EPA says that lead stabilizes in five years. So except for fluoride use, any pipes, whether of lead or lead-soldered, should not now be hazardous. The most revealing statistics, though, are the high blood lead levels in 400,000 newborns each year. Newsweek in its article on lead and the threat to children
(15) said that pregnant women passed this toxic substance to their unborn children by eating, drinking, or breathing it. But even though pregnant women do sometimes have weird cravings, it’s not likely more than a tiny percentage would be chewing paint chips, nor would a significant number of them be engaged in renovating old houses. The lead is in the water–and in foods and beverages prepared with the water. The EPA estimates that 10-20% of the lead in children comes from the water.
(16) That agency, which knows of the lead contamination of fluoride products, insists the amount is too small to be of regulatory concern. What they have overlooked, though, is that it concentrates in the body tissues, and over time, would add up to quite a lot. In addition, it becomes concentrated in products processed with the water. The 10-20% directly from the water can easily become three or four times as much.
The EPA lists as health problems caused by lead the following conditions: Interference with formation of red blood cells, anemia, kidney damage, impaired reproductive function, interference with Vitamin D metabolism, impaired cognitive performance, delayed neurological and physical development, elevations in blood pressure.
(17) The agency also suggests lead my be a carcinogen, possibly causing kidney tumors and lymphocytic leukemia.
(18) Furthermore, it’s a known scientific fact that lead poisons the bone marrow.
(19) Surely, then, it would be prudent to avoid even “a little bit of lead,” assuming that’s all fluoridation contributes. But the evidence shows it’s much more than that. Let us tell you a tale of two cities–Tacoma, Washington, and Thurmont, Maryland. Both of them saw significant decline in lead levels only six months after fluoridation was stopped. (In Tacoma, that was due to equipment problems, in Thurmont, it was a temporary ban by the city council.) Tacoma registered a drop of nearly 50%
(20) in Thurmont it was 78%.
(21) To the best of our knowledge, no other explanations were offered. In Thurmont the ban is now permanent.
(22) In Tacoma, we’re told, a battle continues over whether or not to resume fluoridating. We have more points to add. As we’ve already mentioned, the EPA says that lead may be implicated in causing leukemia. A booklet published by the Leukemia Society in 1987 noted that chemicals which damage the bone marrow can cause leukemia. The Book of Popular Science, 1974, pointed out that bone marrow is poisoned by lead. [Link added by us]
(23) Are we to believe, then, nothing is wrong with putting a little bit of lead into the water (from which it will also enter, more concentrated, food and beverages prepared with the water?) The EPA permits lead-contaminated fluorides to be added; they do not require it. Thus, any community, anywhere, could halt the program any time, with the consent of its citizens, who surely would consent if given the facts.
Lead-tainted fluorides are waste products mainly of the aluminum and phosphate fertilizer industries, largely from US companies. But we’ve learned that in some communities sodium fluoride imported from Japan or sodium silicofluoride from Belgium are used. Neither of these nations fluoridates its own water supplies.
(24) (Don’t you get the feeling we’re in the same category as a Third World country becoming a toxic waste dump for others?) In California recently the Attorney General and two environmental groups have sued the makers of brass pumps containing lead which could contaminate water from wells.
(25) But who is suing companies who sell lead-tainted products to cities for their fluoridation purposes? Who is suing the EPA for allowing it? Where are the lawsuits against the US Public Health Service and the Centers for Disease Control for adamantly promoting it? In conclusion, there’s still the matter of lead being leached from old pipes. Anyone who argues that fluoridation had nothing to do with it will have to explain those well-preserved lead pipes from more than 2000 years ago in unfluoridated Roman water. ♦
REFERENCES:
(1) Book of Popular Science, Grolier, Inc., 1974, Vol.7, 63. (2) Ibid. (3) Book of Popular Science, Vol. 3, 167-169. (4) Book of Popular Science, Vol. 7, 62. (5) Encyclopedia Brittanica, 1957, Vol.21, 545. (6) Ibid., 545. (7) Ibid., 546. (8) Ibid., 545A (9) Book of Popular Science, Vol. 7, 63-64. (10) Encyclopedia Americana, 1945, Vol. 1, 456. (11) Encyclopedia Brittanica, 1957, Vol.1, 715. (12) Book of Popular Science, Vol. 3, 39. (13) Textbook of Pediatrics, Nelson WS, MD, WB Saunders Co., Philadelphia,London, 1964, 1557. (14) Newsweek, “Lead and Your Kids,” July 15, 1991. (15) Ibid. (16) Ibid. (17) Federal Register, Bol. 56, No. 110, June 7, 1991, 264. (18) Ibid., 265-70. (19) Book of Popular Science, Vol. 3, 74. (20) Letter from the Tacoma Public Utilities, Dec. 2, 1992. (21) Fluoride Report, newsletter, April, 1994, 5. (22) Ibid. (23) Book of Popular Science, Vol. 3,74. (24) Letter from Tacoma Public Utilities, May 22, 1992. (25) Kansas City STAR, April 19, 1994.
Plumbosolvency Exacerbated by Water Fluoridation
by Dr. Geoff Pain
♦

Note – No fluoride damage to these Roman pipes
– See above.
More Lead In Drinking Water Due to Electric Grounding.
Fluoride is the most acidic and electron negative of all elements. Fluoride aggressively seeks out lead and dissolves it, especially in acidic, soft water.
There is a custom of using water pipes for electrical grounding.
Many older houses are still grounded through water pipes as above.
This accelerates lead corrosion and increases lead in drinking water.
See also ⇓
Rainwater Users Warned Of Lead Risk
Lead Poisoning At Mount Isa And Fluoridation
Lead in Plumbing Products & Materials –
Macquarie University – Australia
LEAD CONTAMINATION FROM AVIATION FUEL
A MORATORIUM ON SILICOFLUORIDE USAGE WILL SAVE $MILLIONS
Chloramine+Lead Pipes+Fluoride = Contaminated Tap water
 ‘Water Fluoridation Targets Black Americans
‘Water Fluoridation Targets Black Americans
FLUORIDE, LEAD AND PIPES

MORE INFO ON FLUORIDES & LEAD ON OUR POST ⇓
— THE DISAPPEARING SPARROW—
♦ Silicofluorides contain more lead than sodium fluoride.
Compared with NaF, SiFs cause more lead to be leached from
brass pipe and fittings and from the lead solder used to solder
copper pipe and cast iron water mains. For all these reasons
SiFs should be disallowed as fluoridation materials.
See also ⇓
Water Treatment With Silicofluorides And Lead Toxicity
Council LEAD Project NSW
MORE INFORMATION ON LEAD & FLUORIDATION – by John MacArthur
11 October 2014 Original ⇓
The fatal attraction of lead
For millennia lead has held a deep attraction for painters, builders, chemists and winemakers – but it’s done untold harm, especially to children. And while it’s no longer found in petrol, you’ve still got several kilograms of it in your car. Element number 82 is one of a handful that mankind has known for millennia.
The oldest pure lead, found in Turkey, was made by early smelters more than 8,000 years ago.
That’s because lead is very simple to produce. It often comes mixed up with other more coveted minerals, notably silver. And once the ore is out of the ground, thanks to its low melting point, the lead can easily be separated out in an open fire.
One place lead has long been mined is the Derbyshire Dales, at the southern end of the UK’s Peak District National Park.
As well as its tourist-friendly natural beauty, the area’s volcanic and limestone geology also provided the perfect conditions for mineralising the lead sulphide ore called galena.
For 100 million years the lead just sat there harmlessly, locked up in the rock. Then, 3,000 years ago, people began to dig it up. And then the Romans arrived. And soon enough boatloads of Derbyshire ingots were being shipped back to the Continent.
The Romans were the first to exploit lead on an industrial scale. Ice cores in Greenland contain traces of lead dust from 2,000 years ago, carried on the wind from giant Roman smelters. One of the largest, located in Spain, was operated by tens of thousands of slaves.
Lead found dozens of uses throughout the Empire. Being apparently insoluble, it was used to line aqueducts and make water pipes – the word “plumber” derives from the Latin for lead, plumbum.
The Romans excelled at plumbing, unfortunately they used lead pipes.
“I think of it as the plastic of the past,” explains Derbyshire lead mining historian Lynn Willis. “It’s flexible, you can cast it into thin sheets, solder it into pipes.”
The metal was malleable and seemingly impervious to corrosion, and so – just like modern plastics – it became ubiquitous. And not just in Roman times.
“In a large house in the 17th Century you might find the table covered with [lead tableware], the cisterns holding the water, the drains, the pipes.”
Lead has a long association with the building trade, providing a waterproof material for roofing, window frames, and for sealing stone walls. And a heavy lump of lead on a string formed the plumb-line builders used to ensure those walls were vertical.
The metal was found to have other magical properties. Lead carbonate, for example, has provided a cheap, durable paint since ancient times. Known today as “flake white”, it was prized by Old Masters such as Rembrandt because of the steadfastness of its colour and the beautiful contrasts it would bring to their oil portraits.
Meanwhile, glassmakers learned that adding in some lead oxide would yield glassware such as wine decanters that would glisten, because the lead refracted the light across a wider arc.
Unfortunately, a leaded crystal wine decanter turns out to be a singularly bad idea, according to Andrea Sella, chemistry professor at University College London, especially if the wine (or sherry, port or brandy) is held in it for a long time.
“The lead slowly dissolves out into the wine itself. The intriguing thing is that you get a compound that used to be known as ‘the sugar of lead’.”
This compound, lead acetate, not only looks like sugar, it also has an intensely sweet flavour, Prof Sella explains.
“One of the curious things is that the drink that you would put into your decanter would over time gradually become sweeter.”
But lead, of course, is also toxic. Once inside the body, it interferes with the propagation of signals through the central nervous system, and it inveigles its way into enzymes, disrupting their role in processing the nutritious elements zinc, iron and calcium.
And so history is littered with examples of people, often unwittingly, enhancing the flavour of their beverages with lead, with horrendous consequences for the health of the end-consumers.
The citizens of Ulm in Germany were plagued by agonising stomach cramps in the 1690s. But it was soon noted at a local monastery that some of the monks, who happened to abstain from drinking the popular local wine, were being spared by God.
The source was eventually identified as a lead oxide sweetener added to the wine – and then eliminated via what was possibly the world’s first formal ban on the use of lead.
In England, these same stomach cramps became known as “Devon colic” after a similar 17th Century outbreak, this time caused by the lead used in local cider presses.
Gout could also be brought on by lead poisoning, and became a hallmark of the English nobility in the 18th Century. The apparent cause this time was the 1703 Methuen Treaty between England and Portugal, better known as the “Port Wine Treaty”.
It cemented military friendship and favourable trade terms between the two nations, stimulating a booming trade in port. Guess what the wine came laced with? Lead acetate.
Lead-induced gout was all too familiar to the Romans too.
They associated it with the morose god Saturn, who ate his own children.
The link was apt. Chronic lead exposure causes depression, headaches, aggression and memory loss. It can also cause sterility, and some suggest this explains the common failure of Roman aristocrats, such as Caesar Augustus, to produce a natural heir.
How were the Romans poisoned? Tiny amounts of lead in water pipes dissolve into soft water (the lime-scale from hard water stops this process). The Romans also handled lead in the form of coins, pots and dishes. And they used it in paints and cosmetics. However, the biggest probable source was once again wine, specifically a sweetener-cum-preservative the Romans called sapa or defrutum.
The Romans boiled concentrated grape juice down in lead pots into a syrup that helped extend the life of wines. Why lead pots? According to the winemaker Columella, “brass vessels give off copper rust, which has an unpleasant flavour.”The outcome is clear from bones in ancient Roman cemeteries, which contain lead levels more than three times the modern safe limit recommended by the World Health Organization.
~ SOME KEY FACTS ON LEAD ~
-
The Babylonians used the metal for plates on which to record inscriptions
-
Malleable, ductile, and dense, it is a poor conductor of electricity
-
Symptoms of lead poisoning include abdominal pain and diarrhoea followed by constipation, nausea, vomiting, dizziness, headache, and general weakness
-
Resistant to corrosion
Whether this contributed to the apparent madness of emperors such as Caligula and Nero, and the eventual collapse of the Empire remains a contentious question among classical scholars.
But it’s clear that the Industrial Revolution unleashed a new wave of lead poisoning far greater than anything in ancient times, and this time it was the working classes rather than aristocrats who bore the brunt.
Derbyshire lead miners for example were often marked by a black line across their gums – brought on apparently by the chemical reaction between lead in the miners’ blood and sulphur released by bacteria in the mouth, after they had eaten certain kinds of food, including eggs.
The worst affected were those employed in smelting or in the manufacture of
lead-based paints, who found themselves surrounded daily by lead fumes.
 A black line on the gums is one sign of lead poisoning
A black line on the gums is one sign of lead poisoning
Take the Sheffield paintworks, for example. After three months at the works, employees typically developed a skull-like complexion of pallid skin and dark recessed eyes, Willis says. Melancholy, pain, infertility and death followed.
“In the 1870s, the doctor reported that six people out of 70-80 had died the previous year,” says Willis. But he also noted that in his father’s time in the 1830s they had died “like sheep”.
Given that lead poisoning had been around so long, the actions of the chemist Thomas Midgley Jr. appear to have been reckless in the extreme. He is the man who put lead in petrol.
In 1921 as a brilliant young chemist at General Motors he discovered that adding the compound tetra-ethyl lead made engines run more efficiently, eliminating the uncontrolled knocking of early motorcars.
The product was marketed as the benign-sounding “ethyl”. When challenged about the dangers of the lead content, Midgley called a press conference at which he poured the chemical over his hands and breathed in its vapour for a full minute, claiming he could do so every day without ill effect.
In reality, both before and after this incident Midgley spent months plagued by the effects of lead poisoning. GM’s ethyl plant in New Jersey, meanwhile, was forced to close after several workers went mad and some died. The press renamed ethyl “looney gas”. Midgley was a tragic individual.
Later in life he contracted polio and became bed-ridden, so he designed a system of pulleys to raise himself up – only one day he became entangled in them and died of asphyxiation.
However, the greatest tragedy was his legacy. It was Midgley who invented chlorofluorocarbons – CFCs – the refrigerant gases later found to be responsible for opening up the hole in the ozone layer and increasing the incidence of skin cancer. And cars – far more of them than Midgley could have conceived of in the 1920s – would continue to belch out lead bromide fumes for decades.
Although this was a far more dilute source of poisoning than Roman sapa or the fug of a Victorian paintworks, it was incomparably more far-reaching, affecting every city on the planet. And this time the victims were children.
It was another American, the paediatric psychiatrist Herbert Needleman, who was responsible for finally getting the lead taken out of petrol, but not all aircraft.
In the 1970s and 1980s he discovered that even very low levels of lead exposure did irreversible damage to infants, including unborn babies. As they grew up, their IQs were lower, they had trouble concentrating, and often dropped out of school.
As young adults, data suggested, they were more likely to become bullies, delinquents, criminals, teenage parents, drug addicts, unemployed, and so on. Needleman concluded that the lead had permanently
weakened their ability to resist dangerous impulses.
mapped closely to when their respective crime statistics peaked two decades later.
Thanks in large part to Needleman’s work, the US began phasing out tetraethyl lead in 1975, and most of the planet followed suit. Yet it is only now that the possible scale of the harm done by lead poisoning is becoming apparent.
That’s because many academics now believe leaded petrol was responsible for a global crime wave that peaked in the 1990s.
One such is economist Jessica Wolpaw Reyes of Amherst College in the US.
“When we had leaded generations in the 1960s and 1970s, they would have been far more likely to commit crimes, especially violent crimes, in the 80s and 90s,” she says. She found that the timing of when petroleum companies phased out leaded petrol in individual US states between 1975 and 1996.
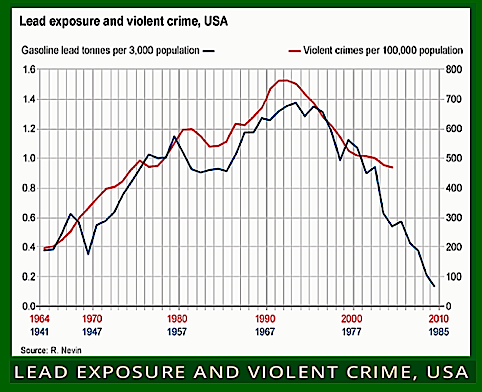
Other studies looking at the difference between countries worldwide found similar results. However, the link between lead and crime is still disputed, with
plenty of other explanations forwarded for the global drop in crime rates.
Continue reading the main story
FIND OUT MORE:
In Elementary Business, BBC World Service’s Business Daily goes back to basics and examines key chemical elements – and asks what they mean for businesses and the global economy.
-
Meanwhile, the drive to eliminate lead from the environment continues. Lead paint is also on the way out. Needleman claimed that it was almost as big a source of poisoning as petrol in the modern world.
All paints, even durable lead-based ones, are prone to crumble eventually. But being a chemical element, the lead never breaks down or disappears. Instead, the dust can be inhaled, or the sweet-tasting flakes can be consumed by a curious toddler.
In the UK the ban has extended beyond bulk household paints to include artists’ suppliers, such as the 150-year-old L Cornelissen in London’s Bloomsbury.
“It is a traditional paint and has passed the test of many, many centuries,” says the shop’s owner, Nicholas Walt, ruefully. “Petrol’s pretty dangerous too, but we’ve learned how to handle it, and it’s a shame that we can’t do the same with flake white.”
Lead can still be found as a radiation shield at your doctor’s surgery, or as a roof lining material in northern Europe. It’s also being used to waterproof and immobilise subsea electric cables for offshore windfarms.
But the biggest use by far is, ironically enough, still in your car. Almost 90% of lead is used to make batteries. Some of them sit in hospitals or mobile phone beacons to provide back-up power in case the grid goes down. But most of them are used to start people’s cars every morning.
Lead is not the most obvious metal for a car battery. Coming from the bottom of the periodic table, it is exceptionally dense, and a great weight to carry around – about as far from a lithium battery as you can get.
However, unlike other batteries, it will provide the initial surge of energy needed to get your engine moving, again and again for years, without breaking. Even hybrid and fully electric cars typically contain a lead acid battery to complement their main lithium or metal-hydride one.
And now for the good news: Unlike a can of leaded petrol, a lead-acid battery is a sealed unit. The lead never escapes. And that remains true even at the end of the battery’s life.
“Lead has the highest recycling rate of any metal,” says Dr Andy Bush, head of the International Lead Association. “The recycling rate in Europe and North America [for batteries] is 99%.”
He says this isn’t just because of environmental regulations.
Lead is a very easy metal to recycle.


That much is clear from a visit to the HJ Enthoven recycling plant at Darley Dale – a last vestige of the Derbyshire lead mining industry.
They take lead batteries, then smash them to pieces in a contained unit. That makes extracting the metallic lead a simple task as it just sinks to the bottom. Lead is also recovered from the sulphurous electrolyte fluid.
All that molten lead is then poured into ingots that can be sent straight back to a battery manufacturer. Even the recovered plastic gets turned back into battery casings.
“It’s a completely closed loop,” says the plant’s manager, Peter Allbutt. “This is a material that is recyclable again and again and again.”
All the same, you may still be surrounded by lead that doesn’t form part of this loop. It remains in some old pipes and in older layers of household paint.
Amazingly, a handful of countries – Iraq, Yemen, Burma, North Korea – continue to use leaded petrol. And there are many more countries in the world, including India and China, which are still getting to grips with the pollution from their lead smelting industries.
And in some places it’s found its way into the earth.
In the Derbyshire Dales, the average lead content in the region’s soil, at 0.05%, is 10 times the UK national average. In some hotspots – downwind from old smelters, or where miners dumped their spoils – it can be as high as 3%.
And it will just continue to sit there, until someone cleans it up.
Faraday observed in the 1830s that lead fluoride when
heated to red hot conducts electricity similar to platinum.
THE SECRET HISTORY OF LEAD
Well put together – recommended reading by us.
Full text → HERE
EXTRACT: …Lead was outlawed as an automotive gasoline additive in this country in 1986–more than sixty years after its introduction–to enable the use of emissions-reducing catalytic converters in cars (which are contaminated and rendered useless by lead) and to address the myriad health and safety concerns that have shadowed the toxic additive from its first, tentative appearance on US roads in the twenties, through a period of international ubiquity only recently ending. Since the virtual disappearance of leaded gas in the United States (it’s still sold for use in propeller airplanes), the mean blood-lead level of the American population has declined more than 75 percent. A 1985 EPA study estimated that as many as 5,000 Americans died annually from lead-related heart disease prior to the country’s lead phaseout…
Fluorosilicates have a unique affinity for lead.
In fact, lead fluorosilicate is one of the most water-soluble forms of lead. Fluorosilicic acid has been used as a solvent for lead and other heavy metals in metallurgy. In industrial applications, chemical engineers rely on this acid to remove surface lead from leaded-brass machine parts. Research shows what happens when we mix it all up. What happens when fluorosilicates in water pass through lead-containing pipes and metal fixtures? Not surprisingly, the fluorosilicates extract high levels of soluble lead from leaded-brass metal parts (researchers from the Environmental Quality Institute of the University of North Carolina-Asheville performed this actual experiment).
Full text ⇒ HERE ⇒ MORE
See also our severn postings below ⇓ ⇓ ⇓
Lead Poisoning At Mount Isa And Fluoridation
http://www.abc.net.au/news/2015-10-06/efforts-to-manage-mount-isa-lead-exposure-questioned/6830098 ← new.
http://www.abc.net.au/news/2015-10-06/mining-town-students-perform-poorly-due-to-contamination/6829792 ← new
http://www.ibtimes.com.au/pregnant-womens-exposure-lead-may-have-lasting-effects-can-be-passed-grandkids-1471841 ← new
Water Treatment With Silicofluorides And Lead Toxicity
More Lead In Drinking Water Due To Electrical Grounding
Lead in Aviation Fuel & Bullets